



Trial Co-Chairs Hallie Levin and Joseph Mueller Spotlighted
Learn More

Litigation Department Chair Ron Machen Featured
Learn More

WilmerHale Women: Trial Lawyers
Learn MoreAbout
-

Our Team
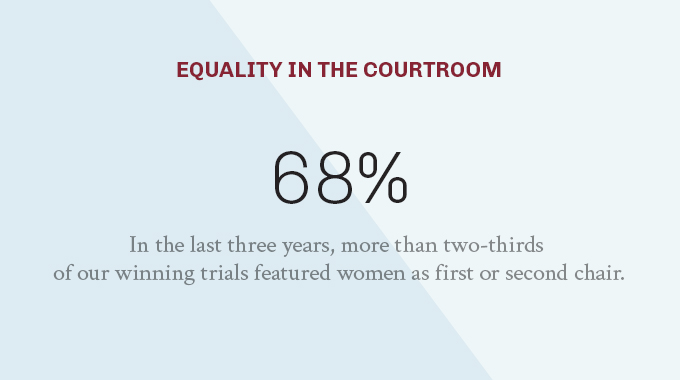
We are proud to field a diverse roster of trial talent—litigators who are as varied in race, gender, ethnicity, geography, and background as they are experienced across legal disciplines, issues, subject-matter areas and jurisdictions.
-

Our Capabilities
We try cases, and we win them. Since 2010, WilmerHale’s Trial Practice has handled more than 320 trials and arbitration hearings in the United States and internationally, many with multiple billions of dollars at stake.
-

Our Approach
We bring clients’ stories to life using classic trial techniques: a relentless focus on themes and narrative, incisive cross-examinations, clean and strong evidentiary records, and a flair for creativity and drama.
-
Decisive Jury Verdict for Creators of Television Thriller in Copyright Infringement Case
-
Gilead Sciences Wins Landmark Patent Trial Against US Government
-
Deaf and Hard-of-Hearing Inmates Win Emergency Alert Access
-
WilmerHale Defeats Billion-Dollar Claims in Apple-Masimo Trade Secrets Fight
-
Victory Preserves Tens of Billions in Revenue for BMS and Pfizer
-
A Novel Trial Approach Saves $26 Billion T-Mobile Merger
Practice Co-Chairs
Featured Recognition
- Recognized by Law360 in the Trials category of its 2023 Practice Groups of the Year awards.
- Recognized by Litigation Daily as a Litigator of the Week winner, runner-up or shout-out nearly 30 times since 2022.
- More than 60 WilmerHale lawyers are ranked in litigation-related areas by Chambers USA and Chambers UK.
- More than 30 attorneys are recognized by Benchmark Litigation. Bill Lee, Hallie Levin and Joseph Mueller were named among the Top 100 Trial Attorneys, and Levin and Sonal Mehta have been named among the Top 250 Women in Litigation and the Top 10 Women in Litigation lists.
- In 2023, The American Lawyer awarded WilmerHale with two Intellectual Property Litigation Department of the Year awards. One for the New England region and one nationally. The firm was also named a General Litigation Department of the Year finalist in the New England awards in 2023 and 2024, and an IP Litigation Department of the Year finalist in 2024. The American Lawyer also named WilmerHale as a Litigation Department of the Year finalist in 2021.
- The National Law Journal named WilmerHale the IP Litigation Department of the Year winner and a General Litigation Department of the Year finalist in the 2024 DC Legal Awards. The firm was previously named Whistleblower Litigation Department of the Year winner and an IP Litigation Department of the Year finalist in the 2023 awards.
- The New York Law Journal named WilmerHale a General Litigation Department of the Year finalist in 2023.
- In 2022, The Recorder named WilmerHale a Tech Industry Litigation Team of the Year finalist as part of the California Legal Awards.







